- Joined
- Jun 4, 2010
- Messages
- 6,642
These are pics I re-linked from an earlier post. ekretz did some incredible camera work.
https://www.bladeforums.com/threads/stropping-wear-resistant-steels-s30v-s90v-cts204p-etc.1372633/
I list the pics in order how they appear in the thread with exception of two diamond/leather stropped pics at the end.
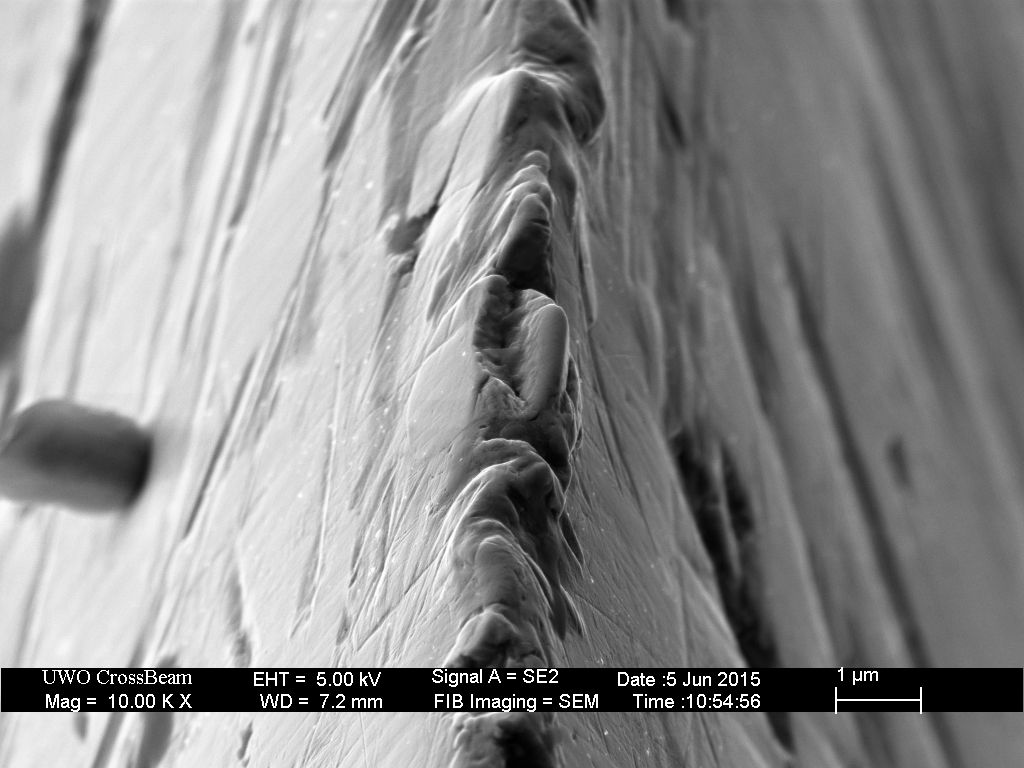
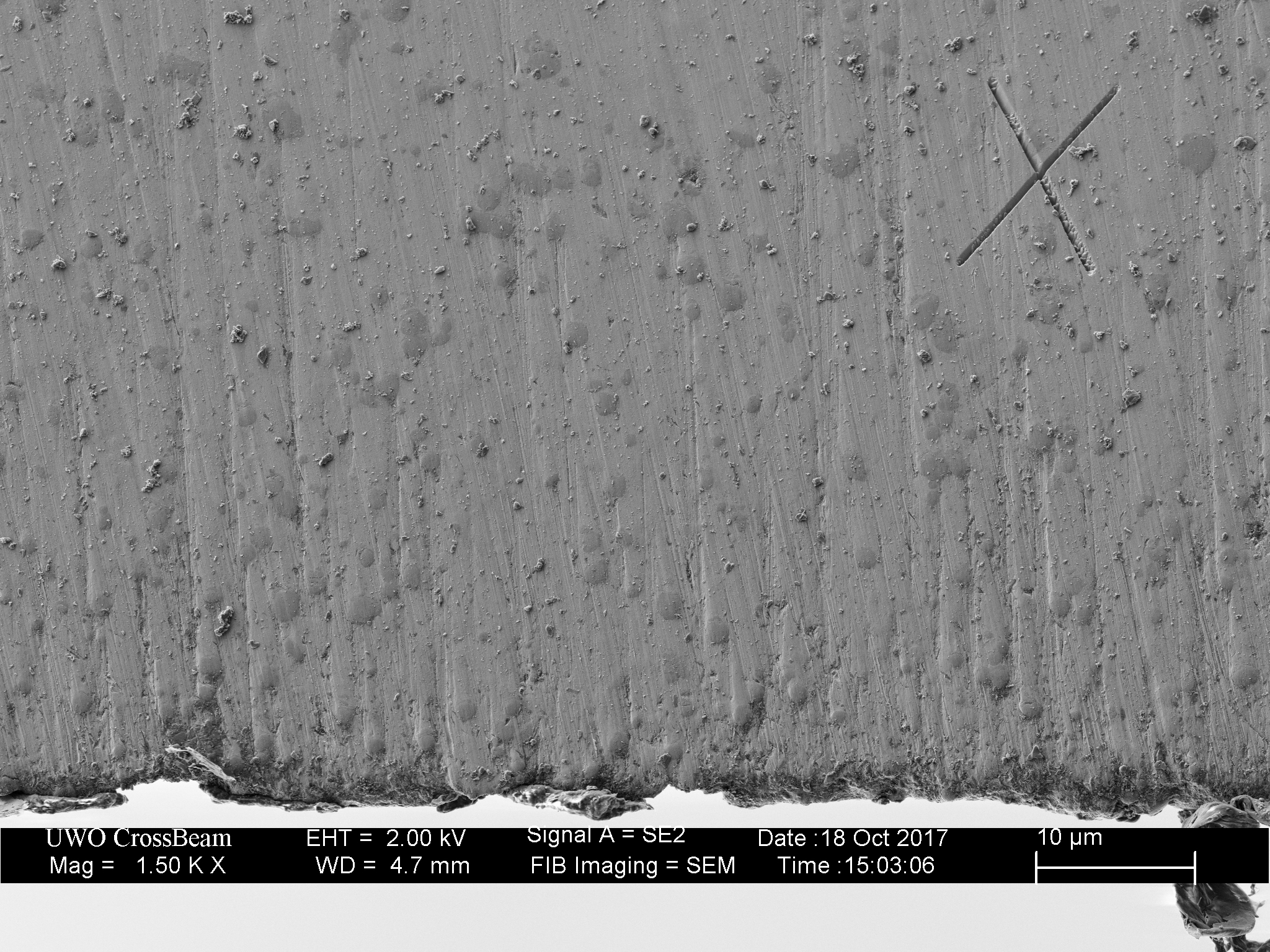
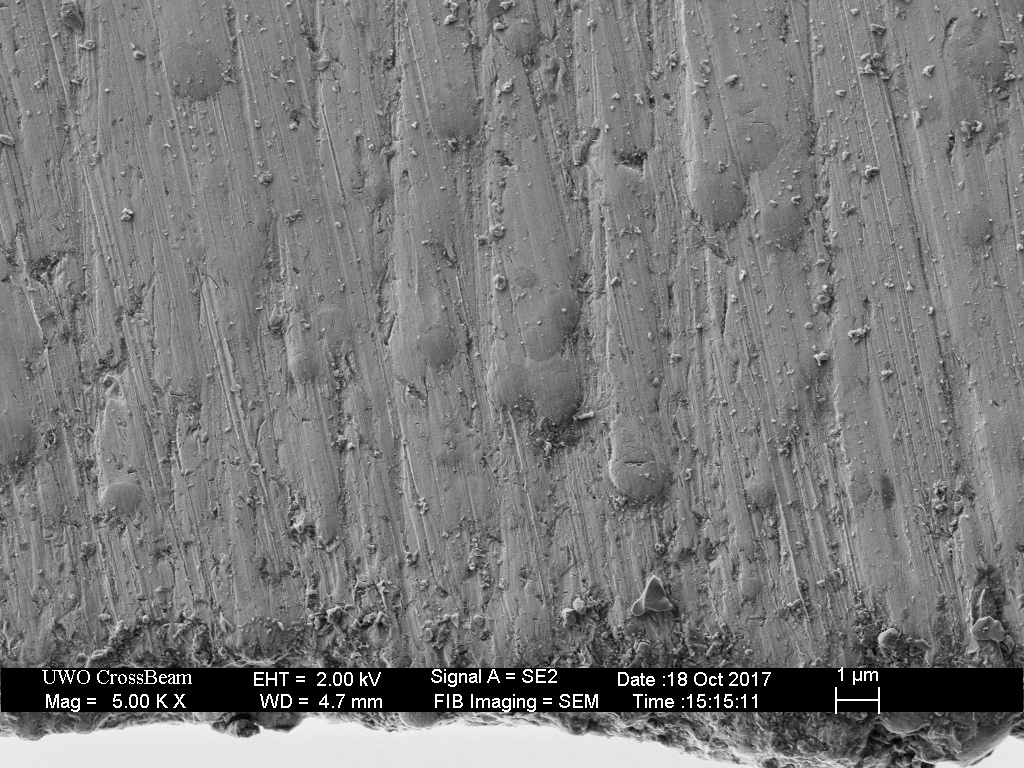
Clearly shows the blown/partially excavated carbides. These are not Vanadium PM steels, but HSS. Still, it clearly demonstrates the possibility if not certainty on some steels of carbide displacement. If anything this would make one want to use diamond abrasive any time the carbide content is significant - I know it changed my thinking considerably.
The DMT EEF is the only fixed abrasive that really did a clean job to the apex, and still some carbides clearly got popped.
HSS with exposed carbides, abrasive used was a Suehiro 20k water stone (Aluminum Oxide abrasive).

DMT C (325)

Next, Shapton Pro 1k:
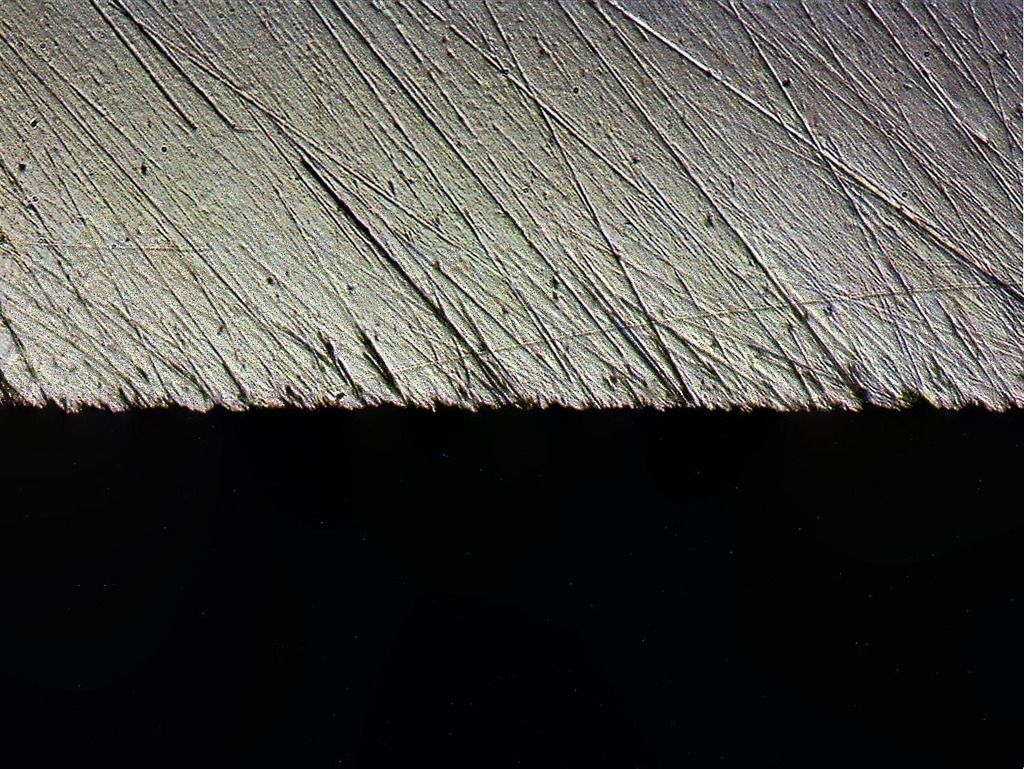
Shapton Pro 2k:
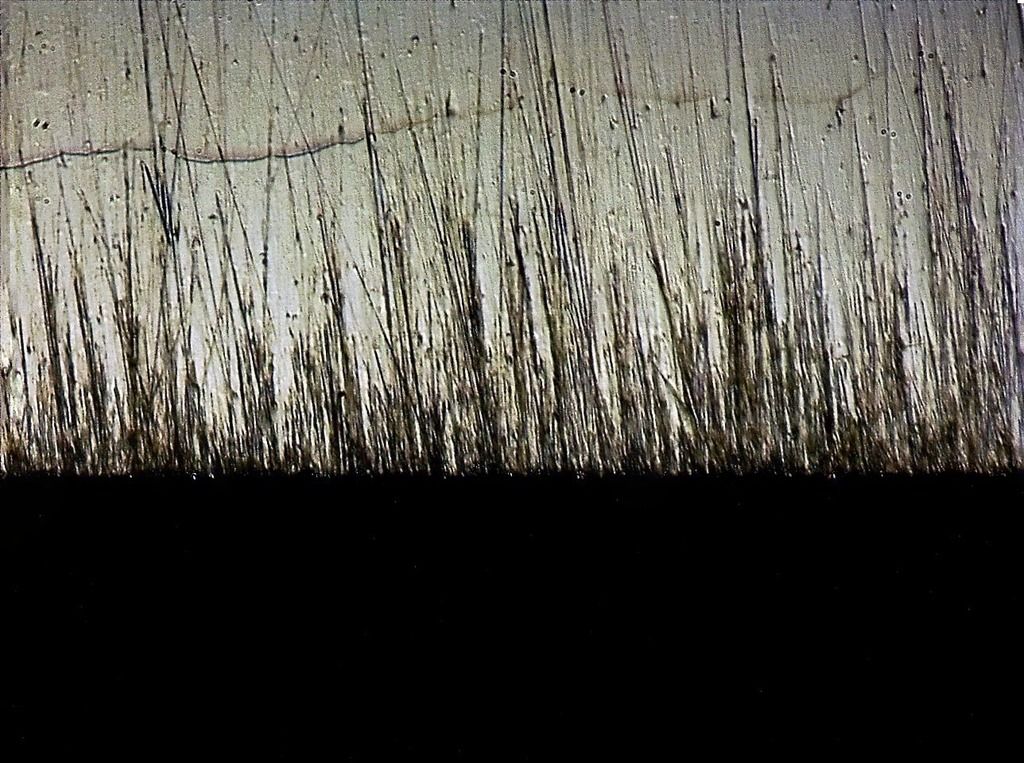
Suehiro Rika 5k:

Shapton Pro 8k:
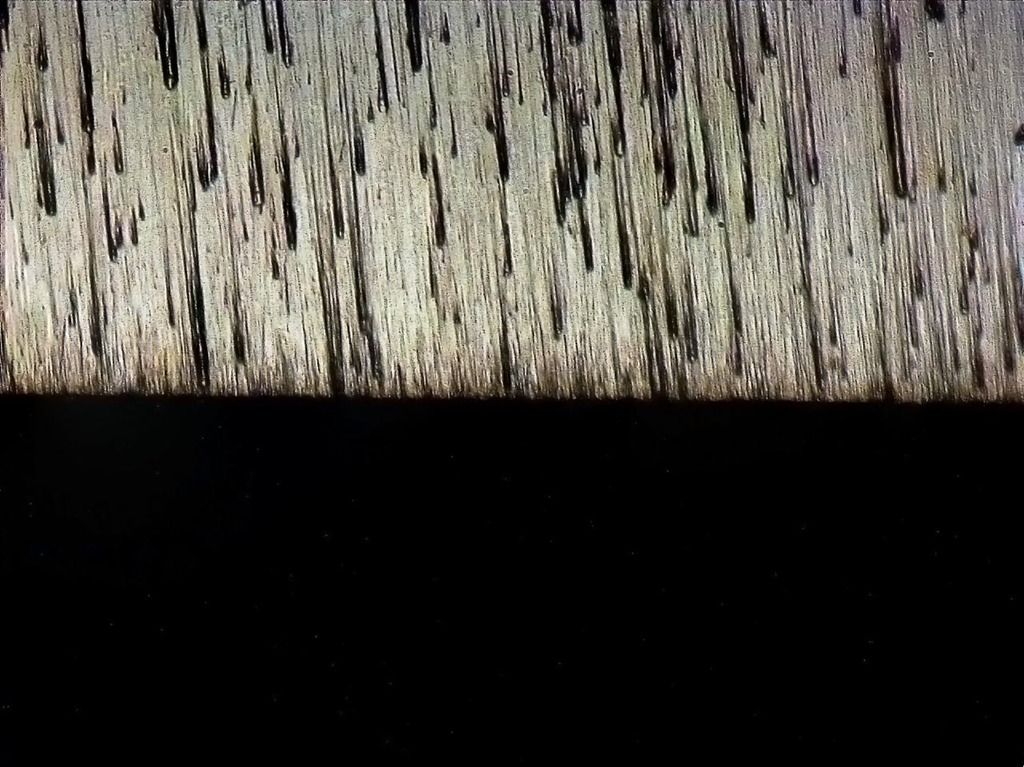
Shapton Pro 12k:

Suehiro Gokumyo 20k:

1 micron diamond compound on leather:

...the finest SiC stone I have, a Carborundum 103

DMT EEF

Spyderco UF
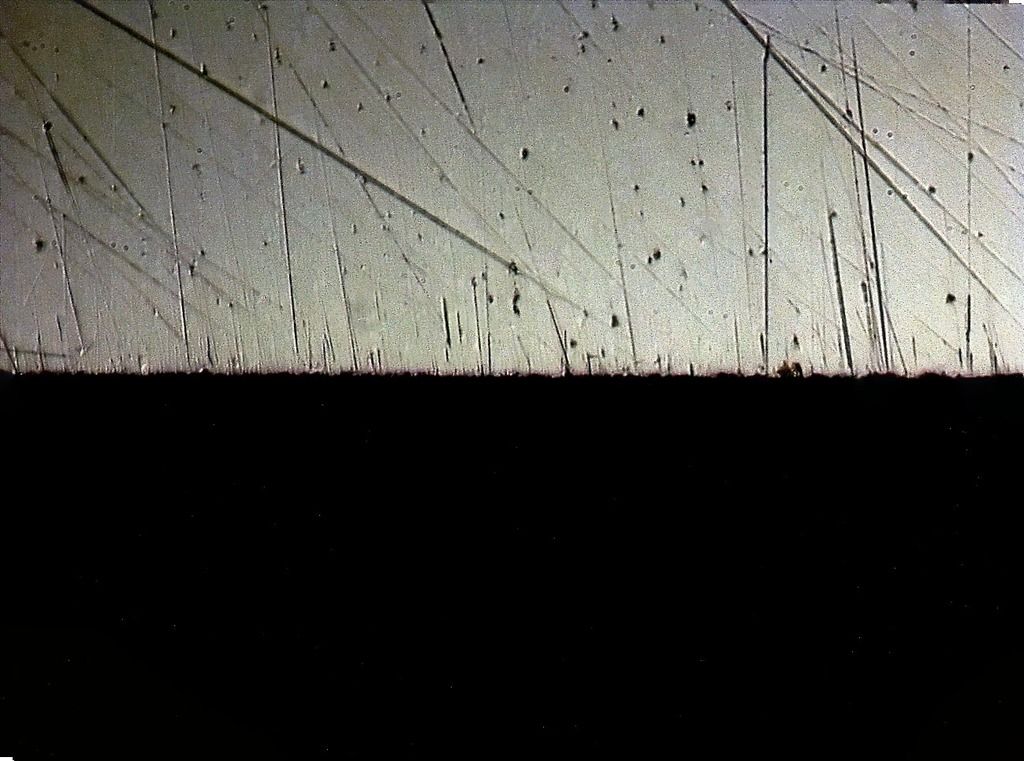
Lapped Spyderco UF
5 micron diamond strop

1 micron diamond strop

https://www.bladeforums.com/threads/stropping-wear-resistant-steels-s30v-s90v-cts204p-etc.1372633/
I list the pics in order how they appear in the thread with exception of two diamond/leather stropped pics at the end.
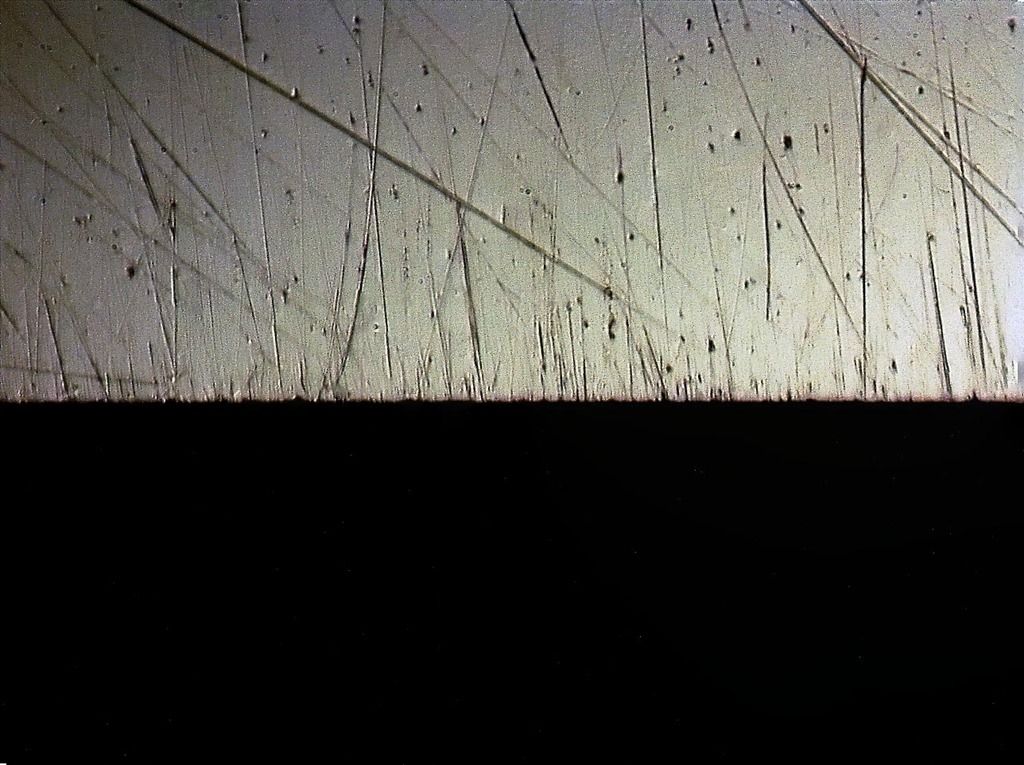
Clearly shows the blown/partially excavated carbides. These are not Vanadium PM steels, but HSS. Still, it clearly demonstrates the possibility if not certainty on some steels of carbide displacement. If anything this would make one want to use diamond abrasive any time the carbide content is significant - I know it changed my thinking considerably.
The DMT EEF is the only fixed abrasive that really did a clean job to the apex, and still some carbides clearly got popped.
HSS with exposed carbides, abrasive used was a Suehiro 20k water stone (Aluminum Oxide abrasive).

DMT C (325)

Next, Shapton Pro 1k:

Shapton Pro 2k:

Suehiro Rika 5k:

Shapton Pro 8k:

Shapton Pro 12k:

Suehiro Gokumyo 20k:

1 micron diamond compound on leather:

...the finest SiC stone I have, a Carborundum 103

DMT EEF

Spyderco UF

Lapped Spyderco UF

5 micron diamond strop

1 micron diamond strop


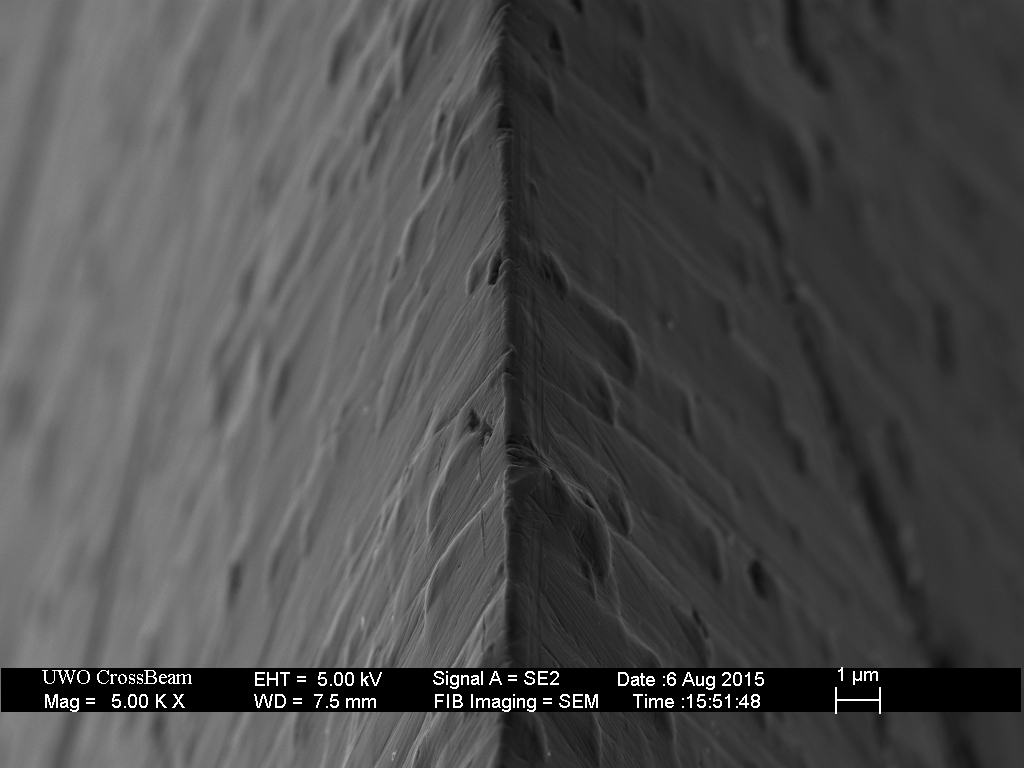

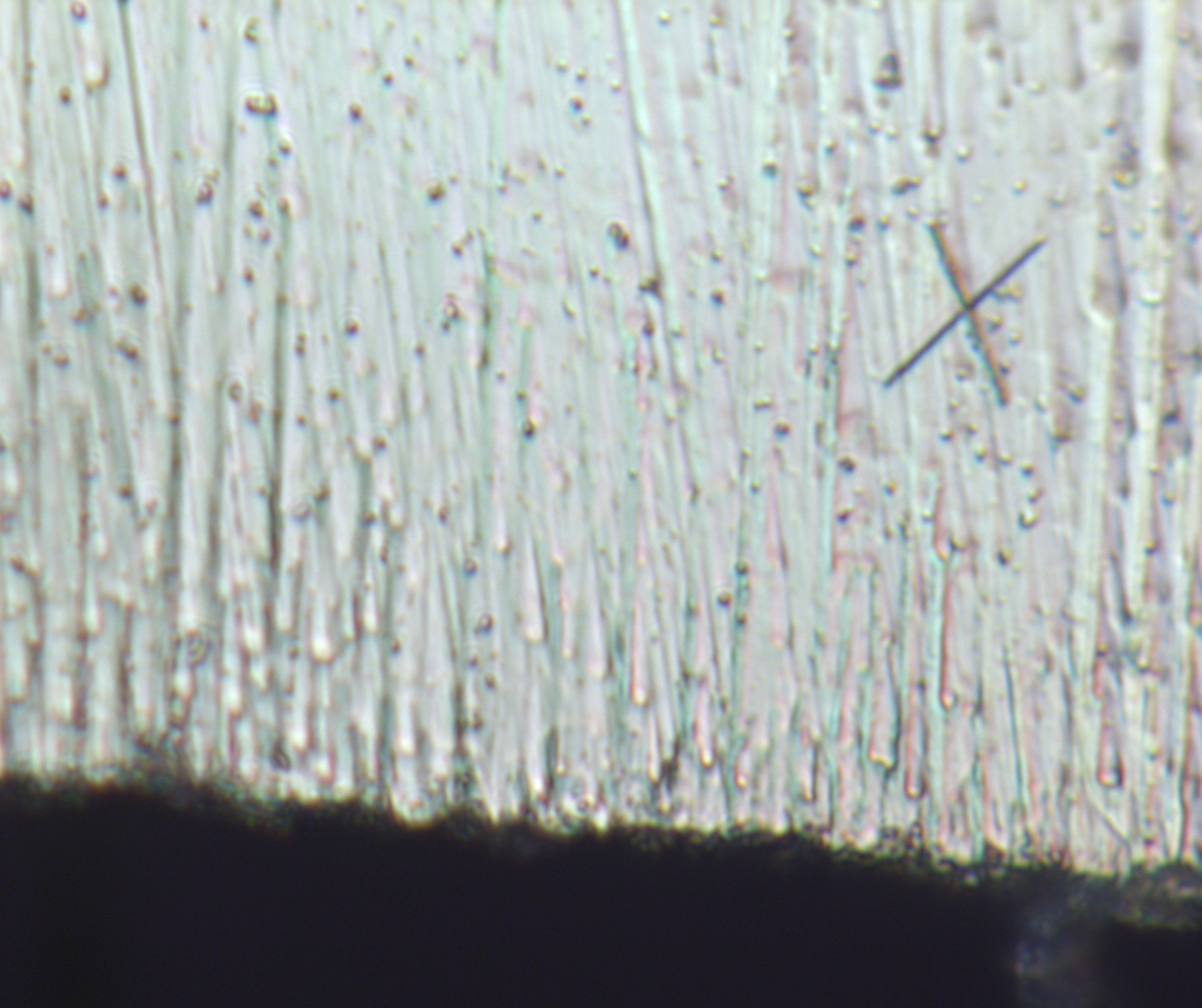
 Also feel free to lmk if my uses of your pics is improper or should treat them as non-public.
Also feel free to lmk if my uses of your pics is improper or should treat them as non-public.