- Joined
- Sep 19, 2001
- Messages
- 8,968
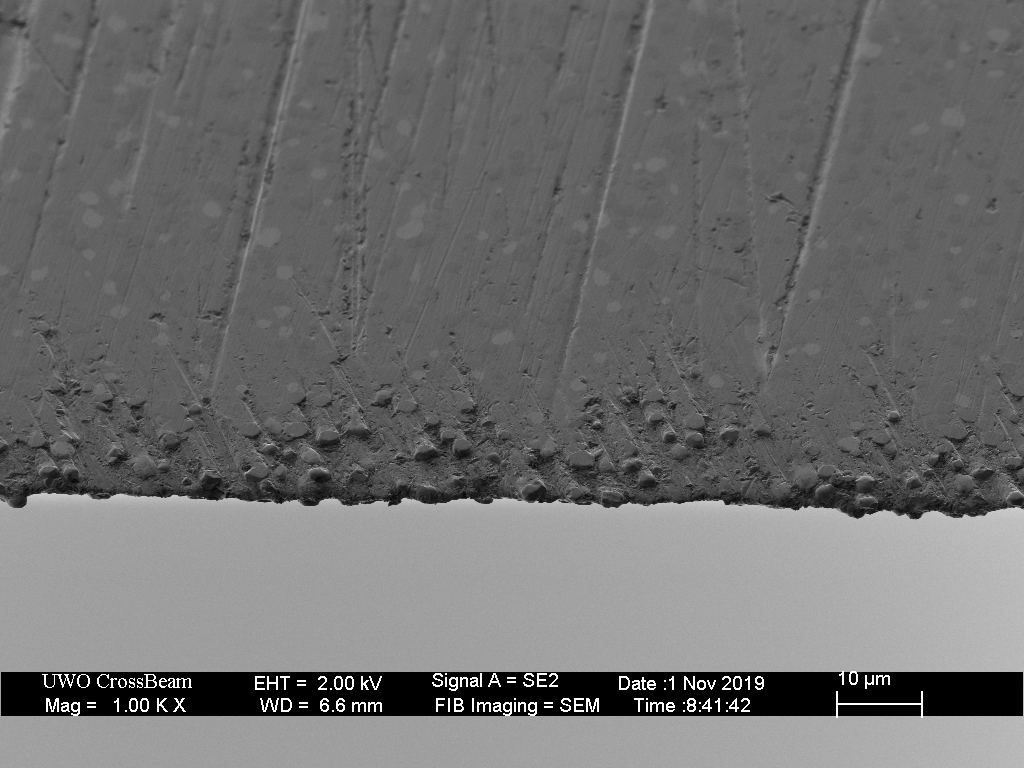
I don't actually use my Rex 121 mule and 15V fixed blades from Farid, though I've had them for years. I was searching for something else when I saw a post about SiC not working on steels above 4% vanadium, which didn't seem to jive with my memory. So I took out my Norton combo Stone from Home Depot and gave it a shot on the mule. I had a weak polish on the edge bevel back when I last played with the knife, though it wasn't sharper than paper slicing.
The SiC scratched the edge bevel well enough, and then when I checked the edge, I was surprised by a meaty burr. I had to work at low pressure on both sides several times to remove it. Then I went to a vintage SiC/carborundum razor hone. That put a hazy polish on the bevel... and raised a small burr again. I chased that one down quickly, then stropped with 1 micron diamond on leather for no good reason because I hadn't really refined the edge.
Has anyone else experienced such burring from Rex 121?
I went ahead and did the 15V on waterstones after, finishing with 13k sigma power. Really minor burr formed at 1k that was easy to remove.
The SiC scratched the edge bevel well enough, and then when I checked the edge, I was surprised by a meaty burr. I had to work at low pressure on both sides several times to remove it. Then I went to a vintage SiC/carborundum razor hone. That put a hazy polish on the bevel... and raised a small burr again. I chased that one down quickly, then stropped with 1 micron diamond on leather for no good reason because I hadn't really refined the edge.
Has anyone else experienced such burring from Rex 121?
I went ahead and did the 15V on waterstones after, finishing with 13k sigma power. Really minor burr formed at 1k that was easy to remove.