- Joined
- Mar 27, 2013
- Messages
- 209
A story tells that when James Black made the legendary knife for Jim Bowie he added a piece of meteorite in a crucible charge. Perhaps in that way he got exceptional Ni steel which was harder and tougher than other steels in his times. Among other things I have touched on this idea in my scientific article “Effect of Ni, Mn, V, and Al on Toughness of Blade Steels” https://www.jstage.jst.go.jp/article/isijinternational/55/10/55_ISIJINT-2014-606/_html
In this current writing I solely focus on the idea of meteorite and Bowie knife.
Bowie knife and meteorite
There are many stories about the metallurgy of the original Bowie knife which was forged by James Black in the beginning of 1800’s. I like most the Paul Welman’s novel “Iron Mistress”. According to the novel James Black melted together steel and meteoric iron in crucible. The story is also dramatized and the meteorite melting scheme is in YouTube
But can a piece of meteorite improve the properties of steel ?
Meteoric iron–nickel
Since prehistoric times man has forged blades from meteorites. Iron meteorites contain Fe, Ni, Co, S, P and C. The amounts of other elements are negligible. The percentage of these elements ranges quite a lot, but the average chemical composition is 0.04C–8Ni–0.5Co–0.2P–0.7P. Most of S is in large visible FeS inclusions, which can be removed, and the inseparable S content of iron is only 0.01–0.001%. I have forged some blades from a fragment of Gibeon meteorite. It was difficult to forge because it tend to fracture during hammering (hot brittleness). Non-hardened specimens were tough, but after carburizing and hardening the specimens were somewhat brittle. It should be note that ancient blades were generally non-hardened and thus meteorites were suitable blade material.
Meteoric crucible steel
According to the story James Black added a piece of meteorite in crucible melt. In other words he made crucible nickel steel. Ni steel has a reputation of good toughness, but in the times of James Black they did not add Mn or Al into steel, which are typical for modern steels.
Without Mn addition S can weaken grain boundaries and result in brittleness, fracture may also occur during forging (hot brittleness). Mn binds detrimental S in harmless MnS inclusions. Old carbon steels, made from pure iron ore and charcoal, had a low S content, and for that reason, the S was not a problem despite the absence of Mn. However, Ni alloying makes steel more susceptible towards harmful effects of S. So, it is a good question, can also Ni steel be tough without Mn addition?
Al alloying prevents grain growth, which makes heat treatment easier, and good toughness can easier be achieved. But can a skilled bladesmith attain a good toughness without Al alloying?
My experiments
Test materials
I made two steels in crucible: totally unalloyed primitive carbon steel and hypothetical Black’s meteoric nickel Bowie steel. And took one factory made AISI 1080 steel which had Al alloying. The chemical compositions of tested steels were as follows:
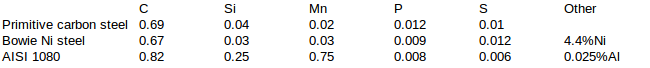
Test methods
I heat treated the specimens to various hardness levels. I used double hardening because it gives finer grain size, and consequently better toughness. After heat treatment I ground the specimens into the size of 3.8x6x30 mm. Then I fractured them and measured the angle of plastic bending of broken specimens (Fig 1.)

Fig. 1 Example of plastic bending angle measurement. Specimen is bent until fracture, two pieces are refitted together, and the amount of plastic deflection indicates toughness.
Results
My test results are shown in Fig 2. When tempered to 57 HRC or softer, all steels were practically equal.
At high hardness levels (64 HRC), AISI 1080 was best, due to Al alloying which resulted in superfine grain size. It had 5 degree of plastic deflection at 64 HRC, which is sufficient for woodcarving, but chopping may need more toughness.
The maximum hardness of un-tempered Ni steel was 63 HRC. In that condition it was brittle, but when tempered to 62 HRC it was tough. An interesting finding was that at hardness level 58-62 HRC the “original” Bowie Ni steel was clearly tougher than the primitive totally unalloyed carbon steel and equal to modern Al-alloyed AISI 1080 carbon steel.
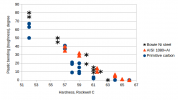
Fig 2. Plastic bending vs. hardness for fracture bent specimens of primitive carbon steel, Ni alloyed “original” Bowie knife steels, and AISI 1080 steel with Al-alloying. Large amount of plastic bending indicates good toughness.
Explanation of the results
The main reason for poor toughness of blade steel is brittle fracture along former austenite grains. During hardening impurities and thin carbide film forms on austenite grains; therefore, martensite tends to fracture along the former austenite grain boundaries.
Al-alloyed AISI 1080 was tough because its fine grain size prevented the brittle grain boundary fracture mode. Primitive carbon steel had larger grain size, and for that reason, when tempered at low temperatures, it suffered grain boundary fracture. But high temperature tempering destroyed the carbide film on austenite grains, and brittle fracture mode vanished. Thus, at 57 HRC primitive carbon steel was as good as modern AISI 1080 despite its slightly coarser grain size.
Obviously Ni alloying also prevented the brittle grain boundary fracture, and for that reason, Ni steel attained the good toughness.
However, we must remember that the effect of Ni depends on the impurities, particularly S. A Mn containing steel with 8% Ni is very tough (I have tested it), because S is binded with Mn. Gibeon meteorite have also 8% Ni, but I did not get good result after carburizing and hardening. Obviously high Ni combined with impurities, particularly S, resulted the brittleness. I found with an electron microscope that the fracture mode was a brittle grain boundary fracture.
If James Black added in crucible such kind of meteoritic iron–nickel which contained only a low amount of P and S impurities, he perhaps got similar material to my “original” Bowien Ni steel. Thus, it could be possible that the original Bowie knife was made of pretty good Ni steel.
Conclusion
My study suggests that, as Paul Wellman’s story tells, it could be possible that James Black achieved superior mechanical properties for Bowie knife by melting mixture of steel and meteorite in crucible.
http://www.juhaperttula.com/
In this current writing I solely focus on the idea of meteorite and Bowie knife.
Bowie knife and meteorite
There are many stories about the metallurgy of the original Bowie knife which was forged by James Black in the beginning of 1800’s. I like most the Paul Welman’s novel “Iron Mistress”. According to the novel James Black melted together steel and meteoric iron in crucible. The story is also dramatized and the meteorite melting scheme is in YouTube
But can a piece of meteorite improve the properties of steel ?
Meteoric iron–nickel
Since prehistoric times man has forged blades from meteorites. Iron meteorites contain Fe, Ni, Co, S, P and C. The amounts of other elements are negligible. The percentage of these elements ranges quite a lot, but the average chemical composition is 0.04C–8Ni–0.5Co–0.2P–0.7P. Most of S is in large visible FeS inclusions, which can be removed, and the inseparable S content of iron is only 0.01–0.001%. I have forged some blades from a fragment of Gibeon meteorite. It was difficult to forge because it tend to fracture during hammering (hot brittleness). Non-hardened specimens were tough, but after carburizing and hardening the specimens were somewhat brittle. It should be note that ancient blades were generally non-hardened and thus meteorites were suitable blade material.
Meteoric crucible steel
According to the story James Black added a piece of meteorite in crucible melt. In other words he made crucible nickel steel. Ni steel has a reputation of good toughness, but in the times of James Black they did not add Mn or Al into steel, which are typical for modern steels.
Without Mn addition S can weaken grain boundaries and result in brittleness, fracture may also occur during forging (hot brittleness). Mn binds detrimental S in harmless MnS inclusions. Old carbon steels, made from pure iron ore and charcoal, had a low S content, and for that reason, the S was not a problem despite the absence of Mn. However, Ni alloying makes steel more susceptible towards harmful effects of S. So, it is a good question, can also Ni steel be tough without Mn addition?
Al alloying prevents grain growth, which makes heat treatment easier, and good toughness can easier be achieved. But can a skilled bladesmith attain a good toughness without Al alloying?
My experiments
Test materials
I made two steels in crucible: totally unalloyed primitive carbon steel and hypothetical Black’s meteoric nickel Bowie steel. And took one factory made AISI 1080 steel which had Al alloying. The chemical compositions of tested steels were as follows:

Test methods
I heat treated the specimens to various hardness levels. I used double hardening because it gives finer grain size, and consequently better toughness. After heat treatment I ground the specimens into the size of 3.8x6x30 mm. Then I fractured them and measured the angle of plastic bending of broken specimens (Fig 1.)

Fig. 1 Example of plastic bending angle measurement. Specimen is bent until fracture, two pieces are refitted together, and the amount of plastic deflection indicates toughness.
Results
My test results are shown in Fig 2. When tempered to 57 HRC or softer, all steels were practically equal.
At high hardness levels (64 HRC), AISI 1080 was best, due to Al alloying which resulted in superfine grain size. It had 5 degree of plastic deflection at 64 HRC, which is sufficient for woodcarving, but chopping may need more toughness.
The maximum hardness of un-tempered Ni steel was 63 HRC. In that condition it was brittle, but when tempered to 62 HRC it was tough. An interesting finding was that at hardness level 58-62 HRC the “original” Bowie Ni steel was clearly tougher than the primitive totally unalloyed carbon steel and equal to modern Al-alloyed AISI 1080 carbon steel.

Fig 2. Plastic bending vs. hardness for fracture bent specimens of primitive carbon steel, Ni alloyed “original” Bowie knife steels, and AISI 1080 steel with Al-alloying. Large amount of plastic bending indicates good toughness.
Explanation of the results
The main reason for poor toughness of blade steel is brittle fracture along former austenite grains. During hardening impurities and thin carbide film forms on austenite grains; therefore, martensite tends to fracture along the former austenite grain boundaries.
Al-alloyed AISI 1080 was tough because its fine grain size prevented the brittle grain boundary fracture mode. Primitive carbon steel had larger grain size, and for that reason, when tempered at low temperatures, it suffered grain boundary fracture. But high temperature tempering destroyed the carbide film on austenite grains, and brittle fracture mode vanished. Thus, at 57 HRC primitive carbon steel was as good as modern AISI 1080 despite its slightly coarser grain size.
Obviously Ni alloying also prevented the brittle grain boundary fracture, and for that reason, Ni steel attained the good toughness.
However, we must remember that the effect of Ni depends on the impurities, particularly S. A Mn containing steel with 8% Ni is very tough (I have tested it), because S is binded with Mn. Gibeon meteorite have also 8% Ni, but I did not get good result after carburizing and hardening. Obviously high Ni combined with impurities, particularly S, resulted the brittleness. I found with an electron microscope that the fracture mode was a brittle grain boundary fracture.
If James Black added in crucible such kind of meteoritic iron–nickel which contained only a low amount of P and S impurities, he perhaps got similar material to my “original” Bowien Ni steel. Thus, it could be possible that the original Bowie knife was made of pretty good Ni steel.
Conclusion
My study suggests that, as Paul Wellman’s story tells, it could be possible that James Black achieved superior mechanical properties for Bowie knife by melting mixture of steel and meteorite in crucible.
http://www.juhaperttula.com/

