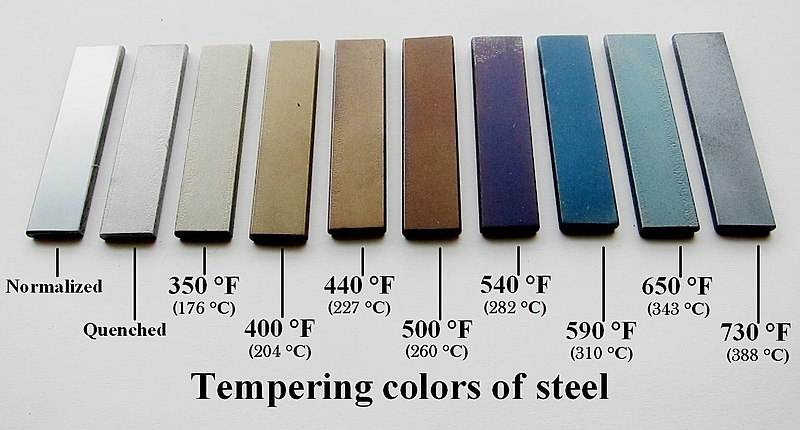
Here is an old post of mine explaining hardening and tempering. It was concerning stainless steel, but the basics are there for carbon steel, too.
It seems clear that many people don't understand what is happening in the steel when it is hardened and tempered. I will try and give a short ( OK, not so short) explanation, but one of the problems ( like yours) from folks who learn on the internet is that reading three sentences and spending three hours trying to learn a skill is not the same as reading three books and spending three years developing the skill. Even that does not compare to reading twenty books, attending twenty seminars, and spending twenty years practicing the skill. I often get a chuckle from the "experts" who are on their third blade.
OK, enough ranting:
Steel is a combination of iron...usually about 99%, and carbon...usually about .6-1.0%.
To that they add small amounts of alloy ingredients. Some are to make it easier to make the steel, such as silicon,phosphorous, sulfur. Others are there to enhance the properties desired in the steel -Manganese, Chromium, Vanadium, Tungsten,and some others.
The alloy ingredients form various structures that are often in the form of carbides, which can make the steel harder and tougher. This comes at a price, though, over a simple steel alloy (with just iron, carbon, and a tad of manganese). Once you start adding things like chromium and vanadium, you need more heat and longer times for these to go into solution.
When the alloy becomes overstuffed with these ingredients, as in martensitic stainless steels, the iron content is reduced to as low as 70% and things like chromium, vanadium, and tungsten go up to as much as 20%. Carbon often reaches nearly the proportions of cast iron ( 2-3%). The excess carbon is needed because of the amount of carbides formed.
What does all this mean to knife makers??????
We want our blades to have a combination of two things - Hardness and Toughness.
This comes from creating a structure in the steel called Martensite. We can then manipulate the martensite into a desired blend of hardness and toughness.
So lets get out steel hot:
When we heat the steel up to a point where the atoms start to rearrange, the first major change comes around 1350F. That point is the Critical point - Ac1 or As,which means it is the beginning of the point where the steel converts to Austenite. Austenite forms at slightly different points depending on the alloy ingredients.The word "critical" means that there is a change in the physical properties (solid,liquid,gas, structure,etc.) at that point.
Next we reach the Curie point at exactly 1414F for steel. This point is the same for all steels. Its abbreviation is Tc. This is not a physical change, but deals with ferro-magnetics. At this point the steel becomes non-magnetic. Of all the "tricks of the trade" that smiths will tell you, this is the only one that is entirely accurate and repeatable. When the blade stops sticking to a magnet...it is at 1414F.
Then we reach the point where the alloy ingredients are able to go into solution. This is the target point. This point is often called Ac3 or Af. NOTE - Charts with the "s" and "f" used are easier to read, I consider that the letters mean "start' and "finish". For this reason, I will use those terms. The target is the place where all that we want to happen will, and things we don't want won't. Grain growth is the enemy when the steel gets too much above the target point.
Engineers have spent many years doing tests on steel to determine what it does and when it does it. Each steel type has its own set of data. This data is put into charts by engineers, because they do that sort of stuff for fun.
A look at one of these funny charts with funny names like TTT or ITC will show a bunch of curved lines. The curve sort of looks like a nose, and the left-most spot is called "The Nose". That "Nose" is usually about 1000F on the chart. This is the point where if your steel goes any amount right of the nose in its cooling ( takes too long to cool down), it will become partly or all pearlite ( which is not what we want). To determine what you want the chart to tell you, look at the base of the chart and the vertical side of the chart. Along the base you will see the time expressed in seconds, and then in minutes. On the vertical side you see the temperature scale.. Thus teh chart shows how soon things happen at any specific temperature . The X/Y coordinates of any spot on the curved nose line will tell you how much time you have to get down to that temperature during quench.
All those funny letters and names assigned to the places along the temperature chart tell you where the steel changes from one structure to another. A= austenite; M= martensite; Ferrite is iron and carbon ( body-centric for those engineer types); Cementite is a hard and brittle structure of iron and carbon; Pearlite is a soft structure made up of layers of ferrite and cementite.
Lower case sub letters, "s and f" mean start and finish. Letters "c and r" mean climbing and returning...or simply heating up and cooling down. Note that things don't always happen at the same point rising and falling.
What we are concerned with is taking a piece of steel that we have shaped into a blade and changing it from one structure to another in a controlled process we call "Heat Treatment".
We do this by first heating the steel up in an environment that won't ruin the blade. This is part the atmosphere of the forge, and part how we protect the blade form elements we don't want to add to our steel....mainly oxygen. That is for another discussion, though, and for this talk we will assume your forge/oven atmosphere is right and you did the proper things to protect the blade form evil oxygen.
continued on next post …...